Multiple Liquid Phases: Miscibility
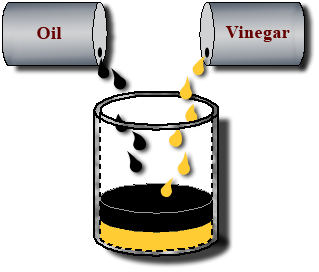
A system can have multiple liquid phases when the substances do not dissolve in each other. Such substances are called immiscible.
For example, oil is not miscible in water. Thus, when oil and water are mixed, two layers, or liquid phases, will exist.
When a system has multiple liquid components that completely dissolve in each other, they are said to be miscible.
The resulting mixture can be considered a pure substance if neither liquid participates in a chemical reaction.

Confused and have questions? We've got answers. With Chegg Study, you can get step-by-step solutions to your questions from an expert in the field. If you rather get 1:1 study help, try 30 minutes of free online tutoring with Chegg Tutors.
© B-Cubed, 2003, 2005, 2006, 2014, 2018. All rights reserved.
Roll your mouse over this box to close.
Join Learn Thermodynamics Advantage
- Download Data Tables
- Download Study Aids
- Homework problem hints and answers
- Get Help from Dr. B in the LT Blog
- 120 day membership
Get it ALL for $5 US
