| 6G-1 : | Efficiency and Coefficient of Performance of Carnot Cycles | 4 pts |
|||||||
| A Carnot Cycle operates between 200°C and 1200°C. Calculate… | |||||||||
| a.) | …its thermal efficiency if it operates as a power cycle | ||||||||
| b.) | …its COP if it operates as a refrigerator | ||||||||
| c.) | …its COP if it operates as a heat pump | ||||||||
| Read : | This is a straightforward application of the definitions of efficiency and coefficient of performance. | ||||||||
| Given : | TH | 1200 |
°C | TC | 200 |
°C | |||
| TH | 1473.15 |
K | TC | 473.15 |
K | ||||
| Find : | η | ??? |
COPR | ??? |
COPHP | ??? | |||
| Diagram : | Not necessary for this problem. | ||||||||
| Equations / Data / Solve : | |||||||||
| Part a.) | The thermal efficiency of a Carnot Cycle depends only on the temperatures of the reservoirs with which it interacts. The equation that defines this relationship is : | ||||||||
| Eqn 1 | |||||||||
| Just be sure to use absolute temperature in Eqn 1 ! In this case, convert to Kelvin. Temperatures in Rankine will work also. | |||||||||
| η | 67.9% | ||||||||
| part b.) | The coefficient of performance of a Carnot Refrigeration Cycle also depends only on the temperatures of the reservoirs with which it interacts. The equation that defines this relationship is : | ||||||||
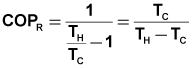 |
Eqn 2 | ||||||||
| Using T in Kelvin yields : | COPR | 0.4732 | |||||||
| This is an exceptionally BAD COPR because it is less than 1. This isn't terribly surprising when you consider that the refrigerator must reject heat to a reservoir at 1200°C !! | |||||||||
| Part c.) | The coefficient of performance of a Carnot Heat Pump Cycle also depends only on the temperatures of the reservoirs with which it interacts. The equation that defines this relationship is : | ||||||||
 |
Eqn 3 | ||||||||
| Using T in Kelvin yields : | COPHP | 1.4732 | |||||||
| This is an BAD COPHP because it is just barely greater than 1. This isn't terribly surprising when you consider that the heat pump must put out heat to a reservoir at 1200°C !! | |||||||||
| Notice also that : | Eqn 4 | ||||||||
| This is always true for Carnot Cycles. | |||||||||
| Verify : | No assumptions to verify that were not given in the problem statement. | ||||||||
| Answers : | η | 67.9% | COPR | 0.473 | COPHP | 1.47 | |||
Example Problem with Complete Solution
© B-Cubed, 2003, 2005, 2006. All rights reserved.

